MDA REG. NO. : GD349191102318
EXPIRY DATE : 03/2026
PRODUCT DETAILS
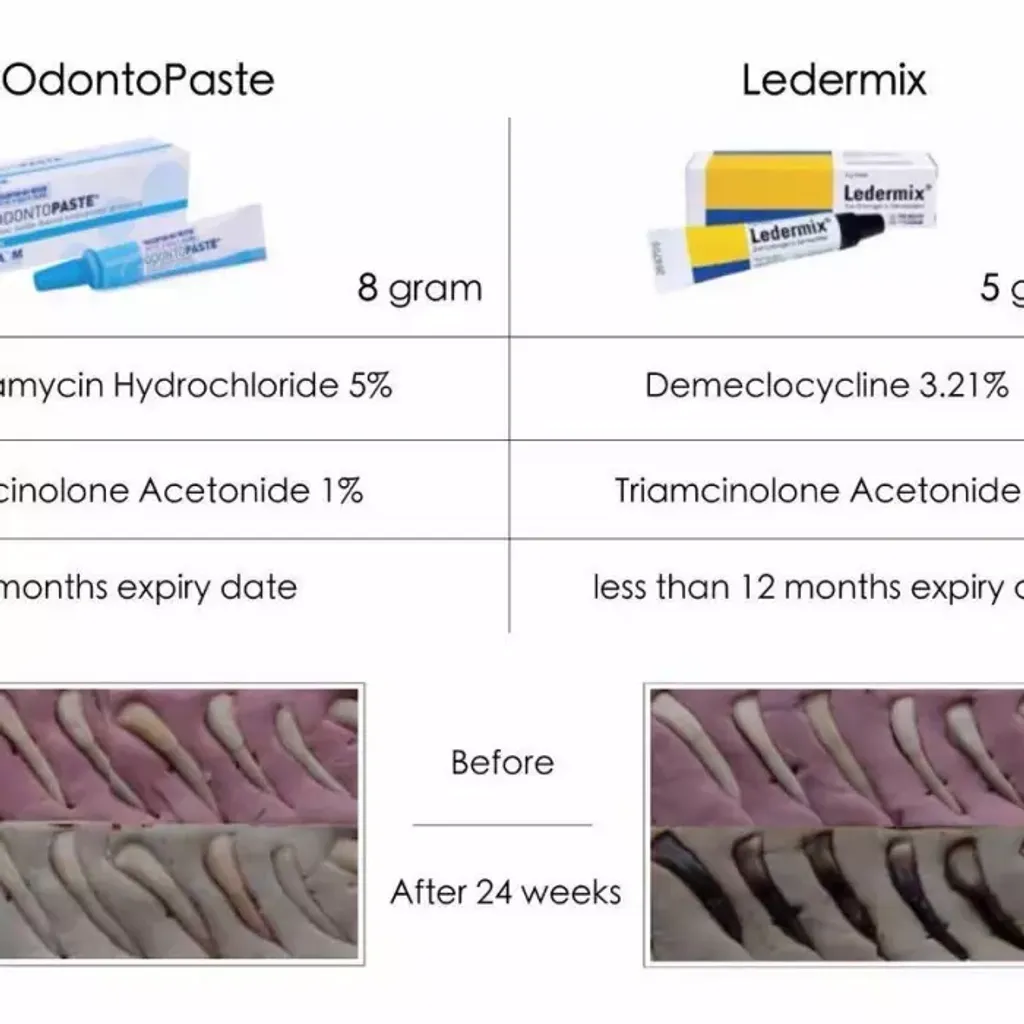
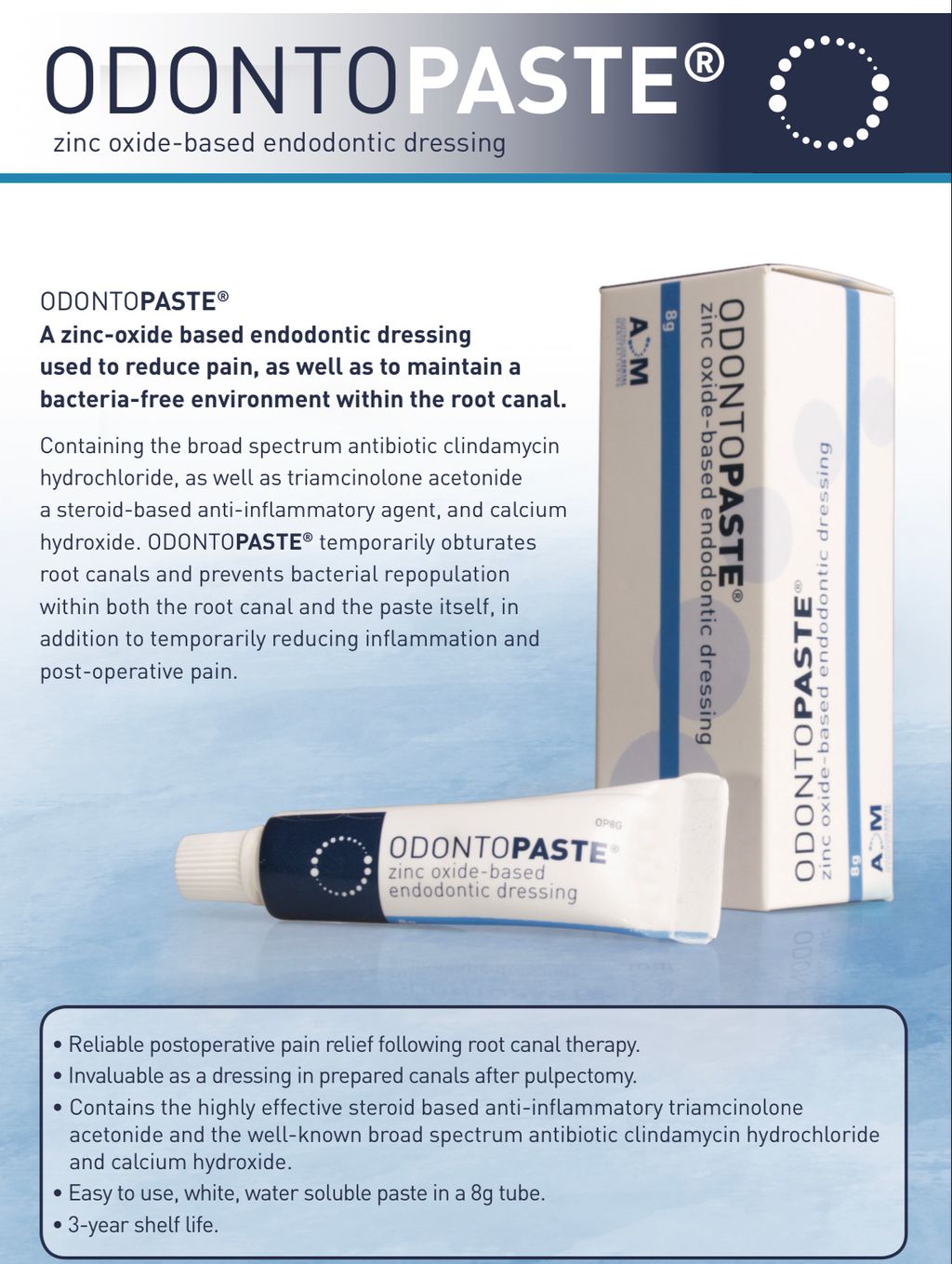
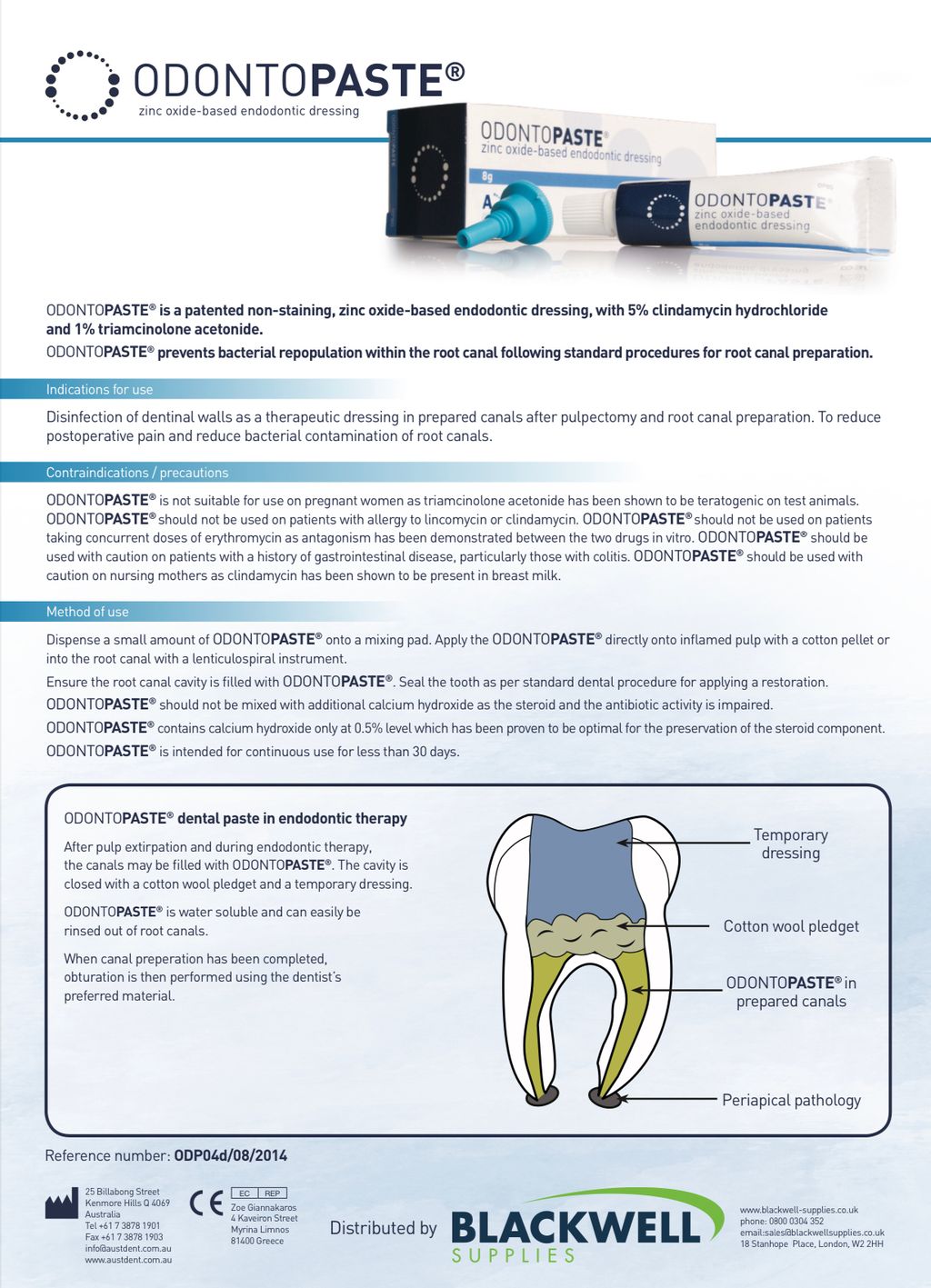
OdontoPASTE is a patented, non-staining, zinc oxide-based endodontic dressing. OdontoPASTE prevents bacterial repopulation within the root canal following standard procedures for root canal preparation.
INDICATION FOR USE
OdontoPASTE is ideal for patients who present in pain and, in particular, for patients with “hot pulps.” OdontoPASTE is also the treatment of choice for avulsed teeth due to its excellent anti-inflammatory properties. OdontoPASTE and OdontoCIDE can be interchanged in teeth should one dressing be unsuccessful in controlling infection or pain. This may be useful in cases of re treatment where teeth are not responding to one or the other medicament.
PRECAUTION
OdontoPASTE is not suitable for use on pregnant women as triamcinolone acetonide has been shown to be teratogenic on test animals. OdontoPASTE should not be used on patients with allergy to lincomycin or clindamycin. OdontoPASTE should be used with caution on patients with a history of gastrointestinal disease. OdontoPASTE should be used with caution on nursing mothers as clindamycin has been shown to be present in breast milk.
METHOD OF USE
OdontoPASTE can be applied in two ways. The first is to dispense a small amount of OdontoPASTE onto a mixing pad. The paste is then picked up with a lentulo spiral and spun down into the root canal. The second is to dispense OdontoPASTE directly into an OdontoTIP capsule and apply into the root canal utilising a standard compule gun. OdontoPASTE cannot be mixed with additional calcium hydroxide as the steroid and antibiotic will be degraded. OdontoPASTE should not be used for longer than 30 days. OdontoPASTE should not be used on patients taking concurrent doses of erythromycin as antagonism has been demonstrated between the two drugs in vitro.
BRAND : ADM (MADE IN AUSTRALIA)
×
×